I picked a couple of proteins out of the PDB to talk about and show a few pictures of.
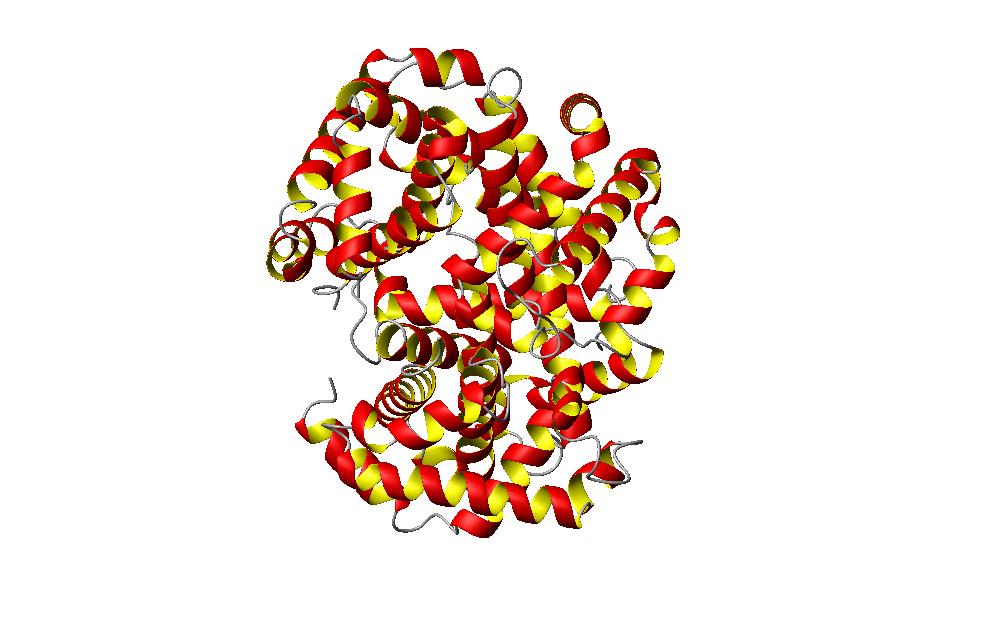
The first picture is a picture of the hemoglobin molecule. This hemoglobin molecule is the unoxidized form of the moleucle, and its PDB identification is 1A3N. Hemoglobin in a molecule found in the red blood cells of vertebrates. This molecule is composed of a protein group and four heme groups. The heme groups are associated with an iron atom. The purpose of the iron atom is to be used in reaction with the oxygen atoms. This allows the protein to circulate through the blood to deliever oxygen to the tissues. After depsoting the oxygen, carbon dioxide is picked up by the protein and carried about to the lungs for respiration. The carbon dioxide is exhaled out as waste. Hemoglobin defects are what cause the disease sickle cell anemia. A close up of the heme binding group on the protein is pictured below. The blue area is where the iron binds.
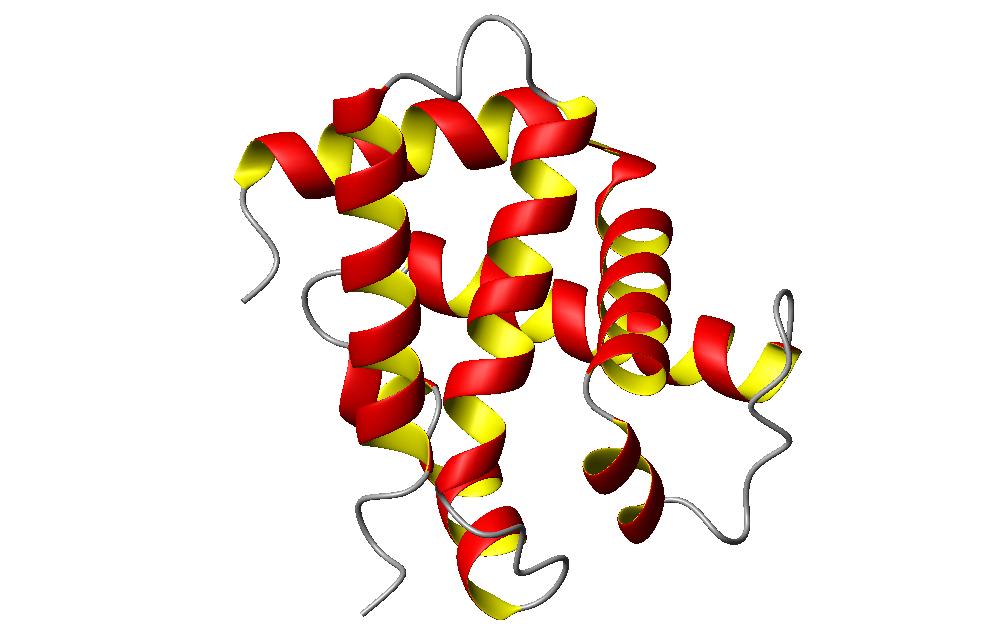
Pictured below is a small segement of the hemoglobin molecule.
I chose this section of the hemoglobin molecule to look at because it shows some interesting characteristics of the alpha helix. Looking at the whole molecule, the protein consists of all helix shapes. This means that the molecule can be classified as an alpha molecule. This small section of the molecule consists of about six sections of helix. In this section, two of the helices on the right side of the secition run mostly parallel to each other. This packing of helices is called the coiled coil. Interestingly, the coil parts of a molecule are usually the sections which can't be classified into any recognizable secondary structure pattern. However, when speaking of alpha helices, the coiled coil represents when two of the helices pack together in a mostly parallel fashion. Looking deeper at the coiled coil pattern, you can see that they aren't exactly parallel, but they form an angle against each other while packing. Also, these coiled coil states create a left handed pitch between the two coils. And while the coiled coils are often created by parallel strands of amino acids, in this case, the two helices are in an anti-parallel formation. The angle of the pitch is around 17 degrees. These coiled coil states form from having 3.6 residues per turn in a helix, and a preference for 3.5 residues per turn in this coiled coil state. The amino acids chains pitch themselves against each other in order to create this simulated 3.5 residue turn.
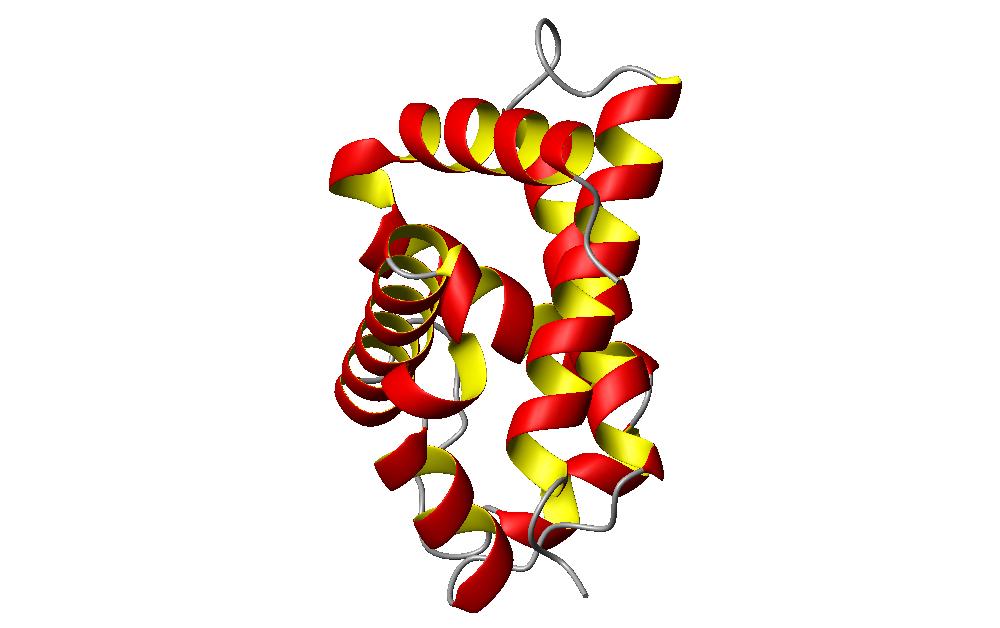
The other noticeable helical packing that is is this segment are the helices that are packed at angles to each other. Actually the same image rotated a bit allows the image to be seen easier.

This section of the hemoglobin molecule shows the other type of helix packing. This picture easily shows the helices packing against each other at about a 50 degree angle. These two formations of helix packing are allowed by the spaces that are created from the helix formation. These can be considered as holes in some instances or ridges and grooves in other instances.
The next protein I downloaded is the reverse transcriptase protein.

This protein has become of interest within the past few years because of its association with HIV. The virus uses this protein to integrate its genetic information into the genome of the host human. This protein has binding domains for the RNA. This protein is a mixed alpha and beta protein because of its groupings of alpha helices and beta sheets. The beta sheets in this protein can be seen easily, and the fact that the sheets aren't generally flat is seen. The sheets curve quite a bit in the protein.
The picture below is the called the p66 thumb of the protein. It is the small area of the protein that is between the main arc of the protein. It consists of three alpha helices and two small strands.
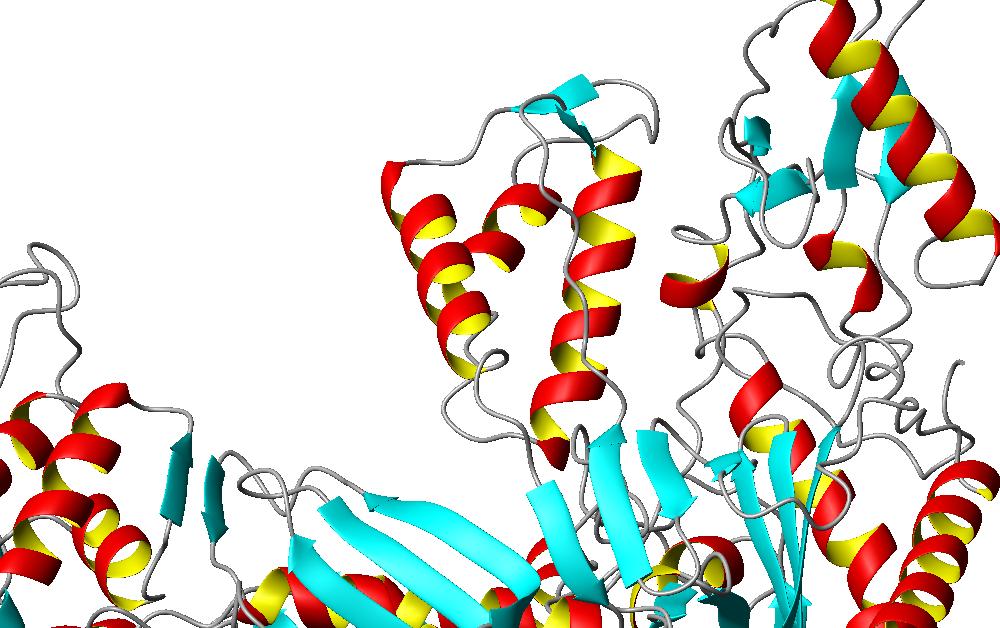
This area of the protein is the most flexible in the entire protein. This is area is rotated around 30 degrees when bound. This thirty degree rotation occurs near the polymerase active site, which is located in the inside arch of the protein. This structural reformation probably allows the protein to access the active site of the protein when bound to the other molecules needed for this process. Checking for these structural changes of the protein can be integral in looking for ways of treating the patients with HIV with different drug therapies.